-40%
US Seller,Digital BC401BT Bluetooth Urine Analyzer+100 Test Strips 11 Parameters
$ 68.11
- Description
- Size Guide
Description
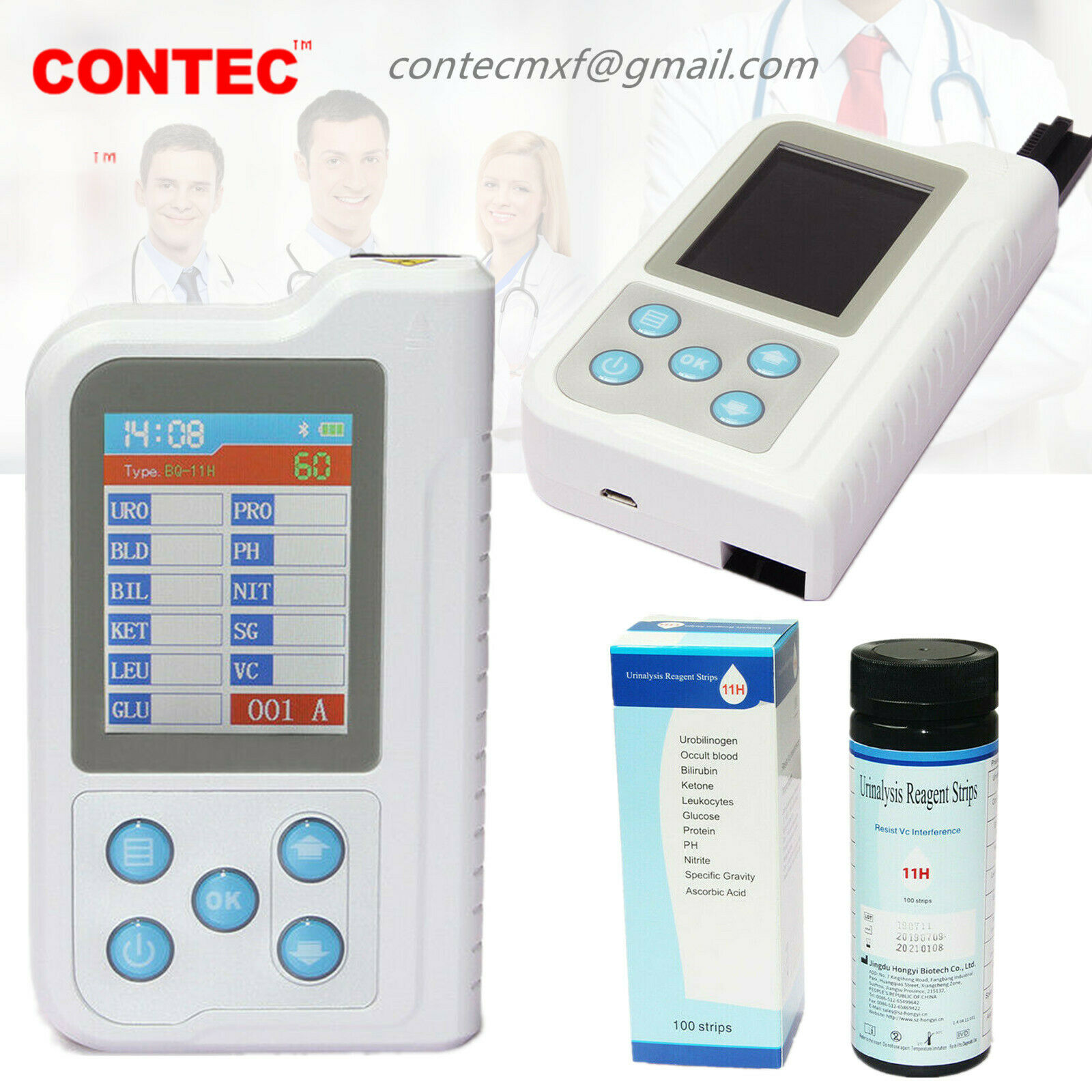
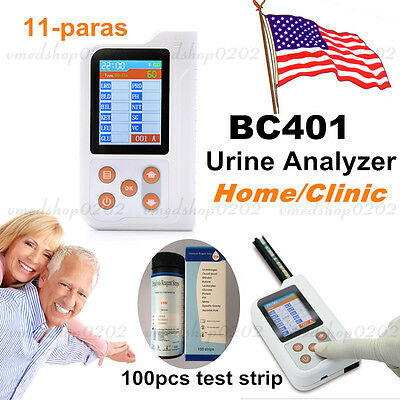
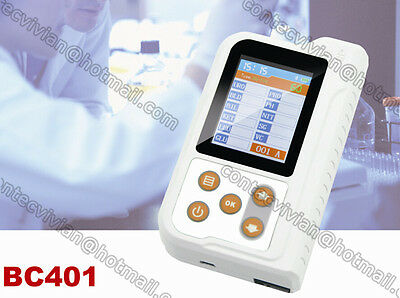
ambulatory blood pressure monitorBC401BT Bluetooth Portable Urine Analyzer+100pcs Test Strips
Description
Brief Introduction:
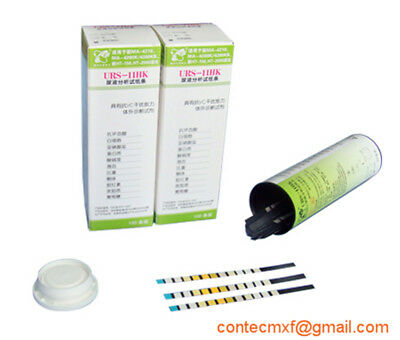
BC401BT Urine Analyzer is a high-precision, intellectual instrument which is researched and developed basing on modern optics, electronics, computer science and other advanced technologies for clinical inspection of urine. GLU, BIL, SG, KET, BLD, PRO, URO, NIT, LEU, VC and PH in urine can be tested by using it with special test strips. And it is applicable for use in hospital, community health service, clinic, epidemic station and family, etc.
Functions:
High-luminance and white LED, features in long life and good stability.
Display abundant contents by the 2.4'' LCD, optional languages: Chinese and English.
User-friendly interface.
Optional units: international unit, conventional unit and symbol system.
Monitoring the whole test process, auto-character and audible prompt.
Be compatible with 8, 10 and 11-parameter test strip.
Standard MicroUSB interface,
Performance:
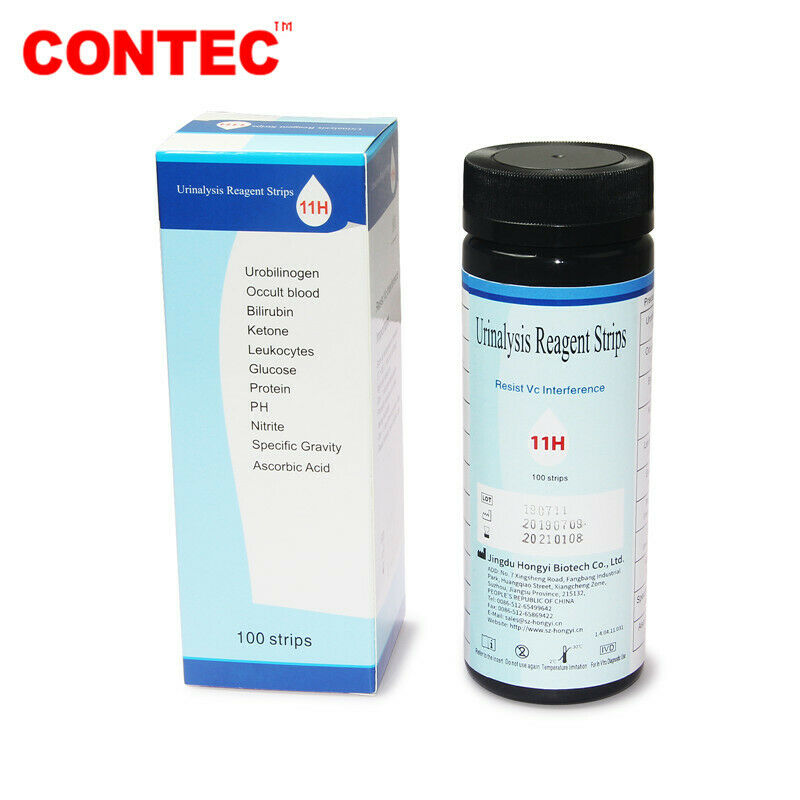
Test items: GLU, BIL, SG, KET, BLD, PRO, URO, NIT, LEU, VC and PH.
Test principle: RGB tricolor
Repeatability: CV≤1%
Stability: CV≤1%
Display: 2.4" color LCD
Working mode: one-step
Test speed: 60 tests/hour
Data storage: Storage of 500 sample data, which can be queried by test date, sample No. and user name.
Interface: Standard MicroUSB interface,
Power supply: DC5V, 1A, built-in rechargeable lithium battery(3.7V 1900mAh)
Accessories:
Power adapter
USB cable
User Manual
Test strip
Physical characteristic:
Dimension:126mm(L)×73.5mm(W)×30mm(H)
Weight: about 0.18Kg.
Payment
We accept Paypal Payment.
Shipping
Clearance: we will ship your item to your confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved.
About Us
Contec Medical Systems Co.,Ltd; 20 Years manufacturer,we have stock in USA and China.
Contact Us
If you have any questions for items,please do not hesitate to contact us through eBay Message, we are on line to reply your message within 24 hours.
Whatsapp/Wechat:+86 18716007715; contecmxfATgmail.com; Skype:contec.tessy